In this article, we'll take a 'deep dive' in to the corpuls acute coronary sysndrome (ACS) algorithm and explore what goes on inside the corpuls defibrillators.
The classic 12-lead ECG represents a snapshot of the electrical activity of the heart, making it possible to diagnose various rhythmological and structural diseases of the heart. Due to this fact, the 12-lead ECG is the most important diagnostic tool for myocardial ischemia and infarction and is the basis for further therapeutic and diagnostic measures (Wagner et al. 2009).
Myocardial infarction (MI) leads to muscular injury (scarring) and consequently to a change in the conduction properties in the heart. This injury, as it changes the hearts conduction properties, can be seen in the ECG trace, for example an elevation or depression of the ST segment, changes in the QRS complex or an inverted T-wave. The ST segment changes result from the voltage differences at the borders between ischemic (damaged) and non-ischemic tissue.
Due to the bioelectrical principles of the ECG, an ST segment elevation in one lead usually leads to a reciprocal ST segment depression in the lead with opposite polarity. Consequently, an ST segment elevation in the posterior leads V8 and V9 can be seen as an ST segment depression in the reciprocal leads V1 and V2 (Wagner et al. 2009).
As the posterior leads are not part of the standard 12-lead ECG, a distinction is normally made between two classes of ACS (acute coronary syndrome). Those are, STEMI (ST-elevation myocardial infarction) and NSTEMI (non-ST-elevation myocardial infarction). The STEMI typically has an ST segment elevation at the J point (the junction between the termination of the QRS complex and the beginning of the ST segment), while the NSTEMI shows an ST segment depression in the corresponding leads. (Wagner et al. 2009)
In a STEMI (patients with acute chest pain and ST segment elevation) there is an acute and total occlusion of a coronary artery. These patients must immediately receive reperfusion therapy or fibrinolytic therapy (Steg et al. 2012). Accordingly, patients presenting with acute chest pain without persistent ST segment elevation have NSTEMI. This is a subacute course and must first be confirmed by laboratory diagnostics to then move on to further therapy.
In order to make a well-founded therapy decision, the ECG is the most important option for a quick and safe diagnosis, as well as adequate risk assessment.
Myocardial ischemia remains the leading cause of death worldwide. Even though this trend has decreased in Europe over the past three decades, heart attacks still caused 1.8 million deaths in 2018. This represents 20% of all deaths (Ibanez et al. 2018). In Germany alone, 60,000 people die of an acute heart attack each year. For this reason, the guide-lines recommend an ECG is preformed and interpreted no later than 10 minutes after first medical contact (Kelm et al. 2018; Ibanez et al. 2018).
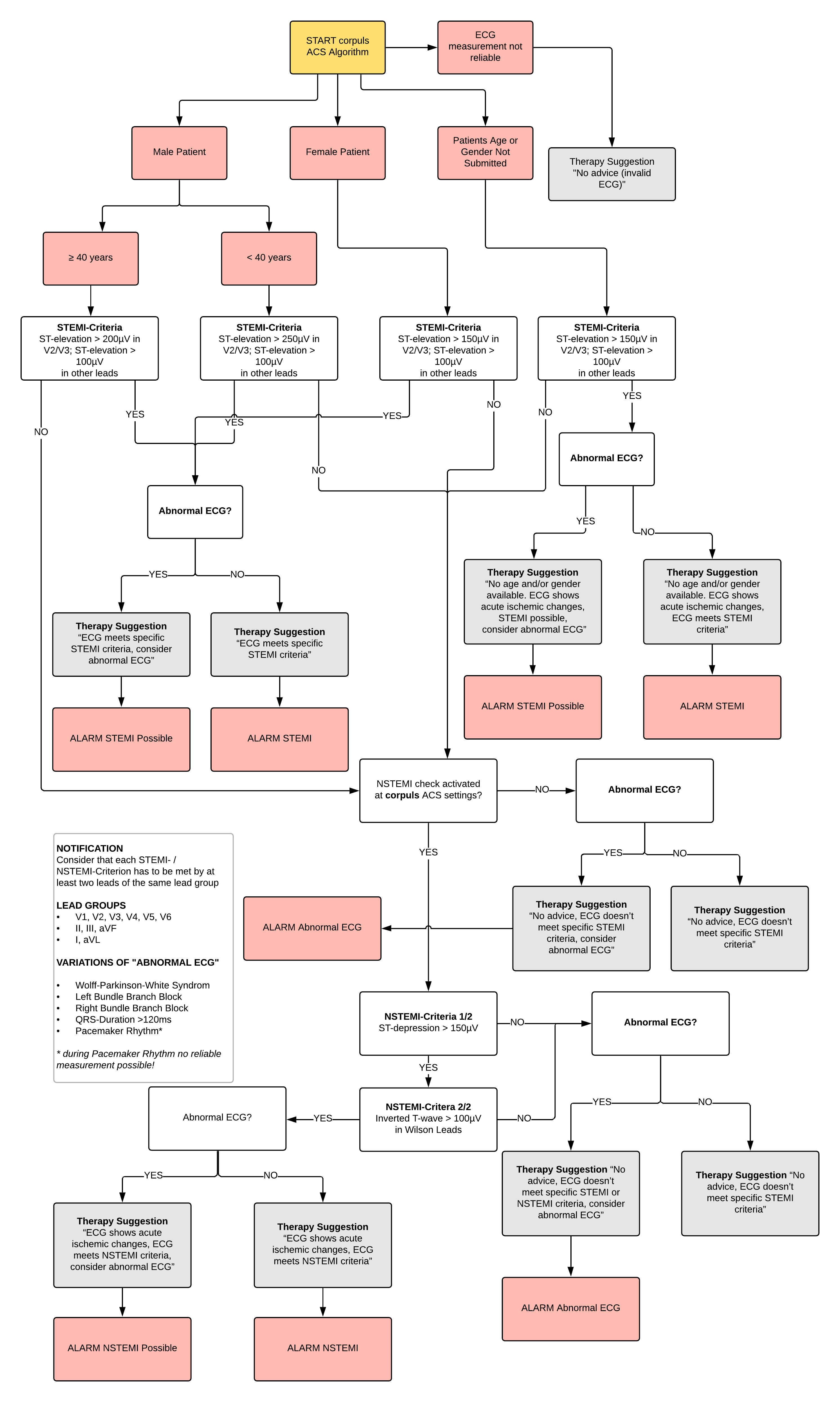
To be able to offer the patient the fastest and best possible therapy, the corpuls3 contains a computer-aided ECG analysis - the corpuls ACS algorithm (built around European Society of Cardiology guidelines). Through this algorithm, the ECG can be analysed according to the STEMI or NSTEMI criteria (see Figure 1).
Fundamentally, the change in the ST segment is measured from the J point. The following criteria are considered to indicate sustained acute occlusion of a coronary artery (STEMI) (Thygesen et al. 2012).
> Two adjacent leads with ST elevation of 0.25 mV in men < 40 years
> Two adjacent leads with ST elevation of 0.2 mV in men > 40 years
> Or two adjacent leads with ST elevation of 0.15 mV in women in leads V2-V3
> And/ or ST elevation of 0.1 mV in all other leads
> In the absence of LV hypertrophy or LBBB
According to the guidelines, an NSTEMI is characterised by an ST depression of 0.05 mV in two adjacent leads. However, as a depression of 0.05 mV (0.5 mm) is usually difficult to diagnose, a segment depression of more than 0.1 mV in a single lead, or even more relevantly by 0.2 mV, is also a clear characteristic (Thygesen et al. 2012; Achenbach et al. 2012; Deutsche Gesellschaft für Kardiologie – Herz- und Kreislauf-forschung e.V. 2011, 2012). For the corpuls ACS algorithm, a segment depression of 0.15 mV was chosen as the NSTEMI limit value. This value should avoid too many false positives yet still recognize an actual NSTEMI.
Furthermore, the T-wave inversion above 0.1 mV is a clear characteristic for a NSTEMI (Thygesen et al. 2012; Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e.V. 2012). The corpuls ACS algorithm also checks this characteristic, as shown on the flowchart (Figure 1).
Nevertheless, NSTEMI is a disease that is very difficult to detect in a 12-lead ECG. Over half of all NSTEMI display either a normal or a diagnostically unusable ECG (pacemaker, bundle branch block etc). ST segment depressions only occur in approximately 20% of patients, whereas a T-wave inversion can be found in around 25% of all NSTEMI patients (Arastéh et al. 2012). Accordingly, the guidelines recommend that a measurement of the posterior and the right-precordial leads should also be integrated into everyday clinical practice as routine (Kelm et al. 2018).
Figure 1: Schematic of the ACS Algorithm
References
Achenbach, S.; Szardien, S.; Zeymer, U.; Gielen, S.; Hamm, C. W. (2012): Kommentar zu den Leitlinien der Europäischen Gesellschaft für Kardiologie (ESC) zur Diagnostik und Therapie des akuten Koronarsyndroms ohne persistierende ST-Streckenhebung. In: Kardiologe 6 (4), S. 283–301. DOI: 10.1007/s12181-012-0436-5.
Arastéh, K.; Baenkler, H.-W.; Bieber, C.; Brandt, R.; Chatterjee, T. T.; Dill, T. et al. (2010): Innere Medizin. Thieme, 3. Auflage
Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e.V. (2011): ESC POCKET GUIDELINES 2011. Akutes Koronarsyndrom ohne ST-Hebung (NSTE-ACS). In: ESC POCKET GUIDELINES, S. 1–56.
Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e.V. (2012): ESC POCKET GUIDELINES 2012. 3. Allgemeine Definition des Myokardinfarktes. In: ESC POCKET GUIDELINES, S. 1–24.
Ibanez, B.; James, S.; Agewall, S.; Antunes, M. J.; Bucciarelli-Ducci, C.; Bueno, H. et al. (2018): 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation. The Task Force for the management of acute myocardial infarction in patients presenting with ST segment elevation of the European Society of Cardiology (ESC). In: European heart journal 39 (2), S. 119–177. DOI: 10.1093/eurheartj/ehx393
Kelm, M.; Kastrati, A.; Nef, H.; Richardt, G.; Zeymer, U.; Bauersachs, J.; Kommission für Klinische Kardiovaskuläre Medizin (2018): Kommentar zu den Leitlinien 2017 der Europäischen Gesellschaft für Kardiologie (ESC) zur Therapie des akuten Herzinfarktes bei Patienten mit ST-Streckenhebung. In: Der Kardiologe 12 (2), S. 145–149. DOI: 10.1007/s12181-018-0237-6.
Steg, Ph G.; James, S. K.; Atar, D.; Badano, L. P.; Blömstrom-Lundqvist, C.; Borger, M. A. et al. (2012): ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation. In: European heart journal 33 (20), S. 2569–2619. DOI: 10.1093/eurheartj/ehs215.
Thygesen, K.; Alpert, J. S.; Jaffe, A. S.; Simoons, M. L.; Chaitman, B. R.; White, H. D. et al. (2012): Third universal definition of myocardial infarction. In: Circu-lation 126 (16), S. 2020–2035. DOI: 10.1161/CIR.0b013e31826e1058.
Wagner, G. S.; Macfarlane, P. W.; Wellens, H.; Josephson, M.; Gorgels, A. P. M.; Mirvis, D. M. et al. (2009): AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology;
.jpg?width=342&height=509&name=OrtusAcademy(YELLOWGradient).jpg)




Leave Comment